 Does Stem Cell Therapy Work For Back Pain? - Regenexx
Does Stem Cell Therapy Work For Back Pain? - RegenexxWarning: The NCBI website requires JavaScript to operate. Stem cell therapy in discogenic back pain Ahmed H. Barakat1Brighton and Sussex University Hospitals NHS Trust, Brighton, UK;Vivian A. Elwell1Brighton and Sussex University Hospitals NHS Trust, Brighton, UK;Khai S. Lam2The London Bridge Hospital, London, UKAbstractThe chronic low back pain has substantial social and economic impacts on patients and health budgets. In addition to the magnitude of the problem is the difficulty of identifying the exact causes of the degeneration of the disk with modern diagnostic and imaging techniques. That said, the current non-surgical and surgical treatment modalities for low back discogenic pain do not meet expectations in many patients and therefore the challenge. The goal of regenerative therapy of emerging stem cells is to treat degenerative disease of the disk (DDD) by restoring the cellularity of the disk and modulating the inflammatory response. Appropriate selection of patients is crucial to the success of stem cell therapy. Regenerative modalities for discogenic pain are currently focused on the use of primary cells collected from intervertebral disks or stem cells from other sources, whether autogenic or allogenic. The microenvironment in which stem cells are cultivated has been recognized as a crucial role in the direction or maintenance of the production of the desired phenotypes and can improve their regenerative potential. This has led to a more specific approach in the innovation of more effective cult techniques, delivery vehicles and scaffolding for the application of stem cells. Although stem cell therapy could offer an attractive alternative treatment option, more clinical studies are still needed to establish on the safety and feasibility of such therapy. In this literary review, we intend to present the most recent in vivo and in vitro studies related to the use of stem cell therapy in the treatment of discogenic back pain. IntroductionChronic low back pain affects approximately 632 million people worldwide with prevalence of up to 68% in adults over 60 years of age (,). It has substantial social and economic impacts on patients and health budgets resulting in the highest economic burden among all musculoskeletal complaints (). This tremendous impact was retracted in a multicenter prospective study that included 8 industrialized countries that found that patients with chronic spinal disorders have a lower quality of life compared to patients with other chronic conditions such as arthritis, chronic lung disease, congestive heart failure and diabetes (). Stem cell therapy for disk degenerative disease (DDD) is a relatively new approach with promising results as an alternative to conventional surgical and non-cooperative regimes (). The purpose of this article is to review existing available evidence and provide a basis for future research. In this article we review the literature and present regenerative therapies of stem cells in DDD, different sources of stem cells and their delivery mechanisms in the degenerate disk, as well as an overview of the challenges facing the implementation of this technique. MethodsA complete search for literature was performed independently by two authors (AH Barakat, VA Elwell) and the results were collided and duplicated. The search for literature was carried out in MEDLINE and EMBASE databases from the creation of databases until January 12, 2019. The following terms of the mesh ("Sperm Cells" [Mesh]) and ("Rear Pain" [Mesh] or "Rear Pain" [Mesh] were used. Thesaurus terms were adapted for different databases. The search was limited to English literature and all non-English documents were excluded. A total of 286 documents were identified, reviewed and evaluated. The studies mentioned here cover the latest developments in this rapidly expanding field. Patogenesis of discogenic pain The exact causes of disco degeneration are complex and difficult to define; they involve aging, genetic predispositions, nutritional factors such as obesity, mechanical trauma, smoking and other related comorbidities (-). The adult intervertebral disk (IVD) is an avascular organ that is based on the passive diffusion of the adjacent endplate vessels for nutrition, resulting in a poor inherent healing potential (,). The centralized gelatinous nucleus (NP) is a proteoglican-rich hypoxic and hydrophyllic structure consisting mainly of type II collagen, while the external lamellate (AF) fibrous ring is mostly a type I collagen (-). The extracellular matrix (ECM) of the disk is formed of water, proteoglycans and matrix proteins. The water content of the disc depends on both the proteoglycan and collagen content, and the proteoglycan provides the pressure of inflammation by water retention and collagen that provides resistance to inflammation. The proteoglycan and therefore the water content of the disk increases in progress from the external AF to the NP. On the contrary, the collagen content of the disk decreases from the outside. Aging is associated with lower water and protein content along with high collagen concentrations (). Historically, there is a progressive loss of demarcation between the NNP and the FA with loss of the transition zone. This is due to a change in collagen synthesis by collagen II NP cells to collagen I with subsequent loss of proteoglycan and dehydration (). The primary proteoglycan of the IVD is aggrecan, is present both in the NP and in the AF and has condroitin sulfate chains and kerathan sulfate linked to a protein core (). With aging, there is a decrease in the length of the condroitin sulfate chain and an increase in the lengths of the keratan sulfate chain. This reciprocal change in chain lengths has been associated with the decrease in oxygen supply to the aging disk. The reason is that, oxidation is a prerequisite for the formation of glucuronic acid necessary for the synthesis of condroitin sulfate however is not for the synthesis of kerathan sulfate. Aggrecan binds to hyaluronan through a bond protein to form proteoglycan aggregates responsible for maintaining the pressure of inflammation. This binding capacity decreases with the age that leads to the decrease of the aggregates and the hyalurona concentration on a degenerating disk (). Another component of the NDE is the metalloprotein matrix (MMPs). MMPs play an important role in the degradation of the NDE within the aging disk as a result of an imbalance in their billing and activation in relation to the loss of their inhibitors (). These MMPs are mainly activated in acid PH of degenerated IVDs due to their decrease in nutrition and the most aerobic metabolism with accumulation of lactate. This decrease in nutrition is mainly due to the growth of the disk size and the calcification of the final plates. After decreased disco nutrition, the cell density of the disk decreases with aging (). During embryonic development, the notochord gives rise to the NP of the disc, while the surrounding AF develops from the mesenchyal tissue. With the aging process, the noochordal cells (NC) in the NP are replaced by mesenchymatous cells of origin, which have a condrocyte and fibroblast appearance. This also explains the poor healing potential with aging like the NCs that have regenerative capacity are exhausted (). The changes mentioned above, including the decrease in the aggregate content of water and proteoglycan along with the growing fibrous nature of the NP cause the disk to be reduced radiographically (). The loss of intrinsic hydrostatic pressure from the disk due to its dehydration affects the disk's resistance to mechanical load (). The result is the narrowing of the disk space, the lumping and eventually the formation of osteophytes with final plaque sclerosis. This causes pressure on nerve roots that lead to back pain and eventually weakness and numbness. With the progression of the IVD degeneration, the disk tends to be vascularized starting peripherally through angiogenesis. Vascular growth eventually extends centrally to the NP and is associated with the inervation of the disk causing discogenic pain (). Another important factor to consider in DDD pathogenesis is biomechanical stress. It is now appreciated that the metabolism of discal cells is enhanced by the physiological compressive intermittent load that increases the production of proteoglynics and MMP tissue inhibitors (). Genetic factors and family predisposition are also etiological factors recognized for DDD. An immunogenetic epidemiological study on 678 patients has strongly involved both genetic and environmental factors in DDD etiology (). This was supported by other epidemiological studies that concluded a strong family predisposition to DDD (). It has been shown that DDD can accelerate in some individuals due to genetic polymorphisms in genes such as aggrecans or MMP genes (). Obesity has been involved in DDD due to biomechanical overload, as well as the metabolic element in these patients. A radiological MRI study of 129 middle-aged men showed a harmful effect of obesity in DDD (). Both non-operational and surgical treatments have failed to meet the expectations of many patients in providing a satisfactory means of managing this condition. In a large-scale controlled study of 1,450 patients focused on the outcome measure [back to work (RTW)], it was revealed that only 26% of RTW patients 2 years after fusion surgery, while 67% of non-operable controls had RTW within 2 years from the date of injury (). Initially, the recruitment of conservative therapy including the use of nonsteroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, opioids and physiotherapy leads to improvement in most patients. However, for patients not responsible after exhausting conservative options, spinal lumbar fusion as a surgical treatment standard can present with significant complications and morbidity (). Complications related to spinal surgery include deep vein thrombosis, infection, and myocardial infarctions that occur in 6.6 per cent of initial surgeries and 6.3 per cent of revisions (,). The adjoining segment disease and proximal junction kyphosis are recognized as a sequel to spinal fusions by means of spinal column mechanics at higher and lower levels of fusion and the resulting abnormal tension during spinal motion (). Other complications include pseudarthrosis and hardware complications. Stem-based therapies have made significant progress over the past decade with numerous clinical trials aimed at providing a non-surgical biological approach to improving pain and function in DDD. In this approach, abnormal conditions of reduced cellularity and altered cell phenotype are the objectives for correction. Patient Selection The goal of stem cell regenerative therapy is to treat DDD by restoring the cellularity of the disk and modulating the inflammatory response. Appropriate selection of patients is crucial to the success of stem cell therapy. The optimal window for the intervention is as follows: early stages of degeneration, lack of response to conservative treatment and before the emergence of the advanced collapse of disk height and hernia (). There is a consensus that ideal candidates for such regenerative therapies should be limited to patients with moderate chronic back pain and disability, as confirmed by functional scores, with a single-level disc affection as demonstrated by the Pfirrmann radiological classification of lumbosacral disk degeneration Grade III or IV on MRI (). The advanced age of donors for stem cells and comorbidities are other factors that should be considered as MSC can show different phenotypic regenerative potentials (,). Cell sourcesThe regenerative modalities of discogenic pain are currently focused on the use of primary cells collected from IVD or stem cells from other sources, whether autogenic or allogenic. Primary Cells Primary and native stem cell studies for DDD have gained interest in the past decade. Several studies have confirmed the presence of NP and AF parent cells in IVD tissues that have multipotential differentiation capabilities (-). These parent cells are believed to be responsible for the regenerative capacity and homeostasis of the IVD tissue, while their age-related exhaustion may be responsible for the loss of this regenerative and restorative capacity of the IVDs. Mesenchymal stem cell markers (MSC) have been identified both in NP and AF. Important parent niches are notochord (NCs) cells known as NP parent cells (NPPCs). Sakai et al. NPPCs identified in the NP tissue through its intimate endothelial kinase (Tie2+) and desialoganglioside (GD2+) surface markers (). Tie2 is a kinase tyrosine receptor expressed in hematopoietic and neuronal stem cells, while GD2 is a plasma membrane marker for the bone marrow (BM) and MSCs of umbilical cord (-). Angiopoietin 1, which is a Tie2 ligand, was found to play a key role in maintaining NPPs and protecting apoptosis cells. This could lead to future research aimed at developing reliable methods to isolate, maintain and expand these parent cells (). As for the AF parent cells, studies have shown that the specific AF parent cells were present in both nondegenerative and degenerative IVDs (). A unique feature of these cells is their potential to differentiate different cell lineages, including adipocytes, condrocytes, osteoblasts, neuronal and endothelial cells. Although the viability of isolating parent cells from pure native disk without fibroblasts and macrophages was demonstrated as a challenge, the incorporation of specific IVD tissue progenitors into tissue-designed scaffolding would significantly affect the regeneration potential and the effectiveness of IVD constructs created in tissue. In order to overcome this difficulty and similar to the techniques of implantation of autologous condrocytes used in degenerated cartilage in other places, autologic isolated disc cells were stimulated in media-conditioning and reprinted in the same degenerated areas from where they were harvested. A canine model demonstrated after 2 years of tracking, persistent cell viability of disk, proliferative capacity, synthetic ECM capacity and proteoglycan content (). The randomized disc trial Euro is a prospective, randomized, controlled, multicenter study that compares the autologist disk condrocyte transplant and the discectomy in 112 patients (). At the time of the discectomy, the autologist disk condrocytes were kidnapped and expanded into the culture then re-injected into the album after 12 weeks. This study showed a clinically significant reduction in low-back pain scores in patients who received autologic discal cell transplants after discectomy compared to those who had dysectomy alone. In addition, the MRI of the treatment group revealed hydration of 41%-discs compared to 25% in adjacent levels that had suffered dysectomy without autologist condrocyte transplant. Mochida et al. () reported that this treatment has proven safety and effectiveness in a 3-year follow-up without significant side effects and with good clinical results. Due to the practical and surgical risks in obtaining primary NP autologist tissue from herniated or adjacent discs, the motivation has also been followed to identify and characterize alternative cell sources for disk regeneration (,). Other accessible cell sources with lower risk of morbidity on the donor site and relative isolation ease, such as the joint and nasal cartilage, have been investigated in vitro and in animal models for NP regeneration (,). These cell sources remain in childhood and more research is required. The MSCMSC transplant has received considerable attention due to its versatility and potential to stimulate a healthier microenvironment of host tissue for its paracritical effects. MSCs are stem cells that have a wide proliferative capacity and multilineal potential in vitro and in vivo (). The effects of MSC on delaying and even investing the degenerative cascade in IVDs have been well documented in many experimental studies, including different animal models before being translated for clinical use (-). Transplanted cells not only restore degenerated IVDs cellular population, but also were able to BDE and aggrecane production, which led to an increase in IVD height (). Multiple tissue sources have been described including BM, adipose tissue, muscle and more recently umbilical cord (-). BM and adipose derivatives (AD) MSCs are the most used sources for this purpose. However, they are usually associated with procedures for the harvesting of cells such as BM aspiration or liposuction. As a result, studies have been conducted on the use of MSC derived from the Wharton umbilical cord and may be a potentially favorable alternative (). A case study was conducted on two patients using human umbilical cord MSCs (HUC-MSCs) with favorable results in relation to pain and disability, as surveyed by visual analog score (VAS) and functional score of Oswestry (). MSCs derived from BM are of particular interest as they are easily accessible and isolated. Therefore, they have been widely studied as a main site and workhorse for MSC isolation for the purposes of DDD treatment (). Currently, alogénic MSCs derived from intradiscal BM are being explored in a randomized phase II controlled study (). IVD single-level degenerate lumbars were selected. Preliminary data show that a greater number of patients treated with intradiscale MSC reported a reduction of ≥50% in low back pain compared to controls at 12 months after injection. Specifically, of patients treated with intra-discal MSC, 69% reported this successful result, compared to only 33% of the patients in control. Studies on autologous stem cells derived from MB have shown a promise manifested by the improvement of clinical results in human trials. In a study of the Orozco et al. () series of cases, autologist MB MSCs were injected into the NP of 10 patients with chronic back pain and followed for 1 year, resulting in an improvement in clinical symptoms without reported adverse effects. Patients were functionally analyzed using VAS, the Occidental Disability Index (OCI) and the 36-Intem Short Health Survey (SF-36). MRI measurements of disk height and liquid content were also performed. There was a rapid initial improvement in pain and disability at 3 months, followed by modest additional improvements at 12 months. The content of the water increased, but the height of the disk was not recovered. In a prospective study, 33 patients with lower back pain and disco degeneration were treated with MSCs produced by culture, self-sufficient, with a follow-up period of up to 6 years (). The study has shown security with only minor adverse events and significant improvements in pain, function and overall subjective improvement as shown by the numerical pain score (NPS), modified single assessment numerical evaluation (SANE) functional qualification and qualification index (FRI). The measurement of the later dimension of the intervertebral disk has shown that 85% of patients evaluated by MRI showed a reduction in the size of the discal lube, with an average reduction size of 23% of the post treatment. In a pilot study of Pettine et al. () twenty-six patients with low back discogenic pain had percutaneous injections of BM-MSCs. There was a significant improvement in VAS and ODI after the 12-month study period with 8 patients who have increased disk heights as evidenced by the improvement in their Pfirrmann MR classification. In another study conducted by Yoshikawa et al. () similar results were reported after 2 years of follow-up. However, the centrifugation process to obtain automated stem cells of BM is limited by the absence of a high concentration of purely homogeneous MSCs. There is adherence to the plastic during preparation (,). One of the methods of emerging separation of stem cells that are not centrifugation is the SELEX cell technique (systematic evolution of ligands for exponential enrichment). SELEX uses aptamers to selectively capture target cells. Atameros are modified nucleic acids identified by large nucleic acid libraries for their high affinity to target specific molecules and cells in likeness to fix antibodies. The concept continues to evolve and is now limited by the specificity and insufficient collection of high purity stem cells. Parent cells derived from other tissue sources such as adipose tissue have also been shown to have significant potential for differentiation and tissue formation capacities (,). The ease of harvesting of stem cells derived from autologist adipose (AD-MSCs) can be performed as an outpatient environment with yields up to 25,000 MSCs per gram of tissue (). This has led the AD-MSC to provide a better alternative and candidate for cell therapy and regeneration of disks, due to their abundance and ease of isolation. In addition, they have a lower inherent capacity for endocondral osification than the MSC derivatives of BM (). In addition, some studies suggested that AD-MSC could be a more appropriate type of cell than BM-MSC for IVD regeneration because AD-MSC could be differentiated in cells with a phenotype more similar to NP (). An in vivo model of mice with severely degenerated IVD treated with AD-MSCs intradiscally also found promising positive radiographic results (). This study showed the survival of AD-MSC injected up to 12 weeks after implantation with a significant increase in aggrecan tissue levels. Another in vivo study on a rabbit model showed that AD-MSC-injected disks exhibited a high secretion of NDEs, as well as the survival of implanted cells 10 weeks after implantation (). A study controlled by the canine model showed a significantly higher production of aggrecan, type II collagen and higher cell density in AD-MSCs treated disks (). A co-cultive in vitro of AD-MSCs with NP cells in a type II hydrogel collagen caused the regulation of the expression of collagen type II and gene aggrecan (). More recently, NCs as stem cells of the pulpit core (NPPC) for regeneration of disks have received considerable interest. NCs are the parents of adult NP cells, and it is thought that their loss in humans during postnatal growth contributes to the beginning of degeneration later in life (). NCs are crucial as they can generate NP cells and can survive better in the unfavorable post-plantation transplant (). Since the NCs are scarcely present in the human tissue of NP adults and cannot be easily obtained as mentioned above, most studies have focused on taking advantage of the therapeutic potential of NC secret factors through co-culture with MSCs leading to an NP more like phenotype (). This could guide future studies to the importance of the generation of functional and high-quality NCs of induction of other multipotent stem cell niches (). The recent approach to the isolation of pluripotent stem cells, such as induced pluripotent stem cells (iPSCs) or embryonic stem cells (CES) could be a potentially promising source for CNCs (). CES are pluripotent stem cells derived from the internal cell mass of embryo blastocyst from an in vitro fertilization in embryo (IVF), while CPS are genetically reprogrammed adult cells to be pluripotent. These cells are different from multipotent MSCs. Sheikh et al. () demonstrated that ESC-derived conndroprogenitors could potentially differentiate into NCs in a rabbit model of IVD degeneration. Compared to CES, the use of iPSCs for disk repair can be more attractive due to patient specificity, less ethical concerns and less immune rejection. Liu et al. () showed some effectiveness of using the natural matrix of the NP tissue to direct the NC differentiation of iPSCs. To promote the differentiation of MSCs, it has been reported that the sectation in the media with climate NC (NCCM) increases the secretion of the glycosaminoglycans (GAG) and the collagen type III and leads to the production of an NP more as phenotype (). Table 1Type of sale Cellular fontFeaturesPulpit parent cells (NPPC) (,)• IVD isolated primary cells• Difficulty in isolating the pure native disk without fibroblasts and macrophages Specific parent cells (AF)• Possible damage to the disk during harvest• Exhaustion and decrease of availability with intervertebral disk cells (IVD) (-)• Autological isolated IVD cells stimulated in medium conditioning and then reprinted• Non-pure parent cells• Possible damage to the disk during the harvestAutomatic syndrome (,)• From more accessible sites such as articular or nasal cartilage• Differences between condrocytes and NP cells• Mainly in vitro studies Mesenchymal stem cells (MSCs)• bone marrow (BM) derived (-)• Bone marrow aspiration need• Absence of a pure homogeneous high concentration of MSCs• Increased inherent capacity for endocondral osification• Adipose tissue derivative (AD) (-)• Liposuction need of adipose tissue• Higher MSC• abundance It could be differentiated in cells with a phenotype more similar to NP compared to BM-MSC• Lower intrinsic capacity for endocondral osification• umbilical cord The Wharton jelly (,)• More feasible insulation and MSC abundance• No need for stem cells to be invasive• Ethical Issues Embryonic stem cell (ESCs) (,)• Internal cell mass of blastocyst of a donated in vitro fertilization embryo• It could be directed to notochord cells• potential tumorosis due to pluripotence• Ethical problems• Allogenic sourcesInduced Pluripotent stem cells (iPSCs)• Genetically reprogrammed specialized cells• Other sources for the production of notochord cells• potential of tumorigenesis due to pluripotence• More specific of the patient (autogen)One of the potential challenges for using stem cells is that they are not differentiated and are transplanting in a hard environment that consists of low cellularity, nutrients and acid conditions (). All of these factors could detriment the potential for differentiation and the feasibility of implanted cells (). Effect of the microenvironment The microenvironment in which stem cells are grown has been recognized as a crucial role in the direction or maintenance of the production of the desired phenotypes and can improve their regenerative potential. Priming stem cells using growth factors or other environmental factors, such as altered oxygen or glucose level, have been subjected to rigorous in vitro and in vivo (-). Several studies have shown that exposure to low oxygen levels increases the differentiation of MSC to NP phenotypes. An in vitro study of Kumar et al. () has shown that MSCs improve the differentiation in NP phenotypes under hypoxic conditions by seculting in a biodegradable polymer hydrogel scaffold. This is consistent with other studies, indicating that the differentiation of MSC depends largely on the local microenvironment (-). In a small-scale human study, the safety and feasibility of an intra-discal injection of autologous, hypoxic microbes and cultivated in five patients with chronic lower back pain (). The follow-up was up to 6 years and used the clinical examination, the RRM radiological evaluation and the quality of life questionnaire as a measure of results to improve. All patients reported overall improvement, there was strength improvement with four of five patient leg mobility. At the end of the follow-up period, no adverse effects or neoplastic transformation were recorded by radiological evaluation. The overall results are promising and could pave the way for a double-blind, randomized, controlled clinical trial with a significant number of patients and the implementation of validated endpoint measurements as next steps to further validate the effectiveness of this technique. Another important factor is the glucose levels used in the cultural environment of stem cells. An in vitro study by Stolzing et al. () on BM-MSCs rats showed that high glucose concentration has a negative effect on the formation of MSC colonies and phenotypic differentiation. This was supported by other authors who have shown that the hypoglycemic conditions in human AD-MSC have a favorable effect on the survival and biological behaviors of these cells (). An in vitro in rat derived from BM-MSC showed improved low glucose matrix biosynthesis and sustained cell proliferation while high osmolarity and low pH conditions reduce biosynthesis and proliferation of MSCs (). High osmolarity has been shown to inhibit the response to DNA damage and the proliferation of detention cells in bovine NP cells. For MSCs, several studies have shown reduction of cell viability and synthesis of matrix by high osmolarity (-). PH was also considered to be harmful to the proliferation of stem cells and the expression NDE. An in vitro study on the cultivation of BM-MSC derived from rats at different levels of PH showed that acid conditions inhibited the aggrecane expression in the NDE, as well as a decrease in proliferation and feasibility and associated with a change in cell morphology (). The differentiation of stem cells is also affected by mechanical tensions depending on the pattern of load and intensity (,). It has been found that the transition and phenotypic maturation of NC residents to cells similar to NP, can be significantly improved by static compression loads or dynamic hydrostatic pressures (,). For example, in vitro studies have shown that the radial compression loads promoted the AF-like differentiation in BM-MSC (), while the differentiation similar to AD-MSC NP can be significantly improved by dynamic compressions (). Although this review focuses primarily on regenerative stem cell therapies, it is worth mentioning the effect of growth factors as part of the microenvironment on improving phenotypic differentiation. The recent understanding of the cellular and molecular cascade of IVD homeostasis has engendered the priming hypothesis of cultivated stem cells or the direct injection of IVD with growth factors such as the epidermal growth factor (EGF) and the recombinant human growth factor and differentiation-5 (rhGDF-5) to improve cell proliferation and matrix synthesis (). RhGDF-5 is a member of the transformative growth factor (TGF-b) superfamily and of the bone morphogenetic subfamily (BMP), and it is known to influence the growth and differentiation of various tissues, including the intervertebral disk. Various in vitro studies on rhGDF-5 have shown that it has a key role in eliminating the degradation of NDE through the suppression of matrice metalloproteas and in improving proliferation and matrix anabolism through increased production of aggrecan, GAG and type II collagen (-). In addition, a study was reported that a combination of rhGDF-5 and hypoxia had a synergistic effect on the differentiation of MSC (such as NP). There are challenges in the direct application of growth factors that cause a sustainable impact on the refurbishment of unreliable tissues. For example, the average life, the interstitial solubility of these factors, the presence of other inhibitors factors, and the low number of viable cells available to be stimulated in already degenerated IVD disks (). A different approach is to try to control the catabolic pathway leading to IVD degeneration. By antagonizing transcription factors that activate proteolytic genes that contribute to the IVD degeneration, such as the nuclear factor-k B (RANKL) ligand receptor activator, the degenerative cascade could be stopped (). The regulation of the expression MMP and ADAMTS has been involved in the destruction of the ECM disk, which has led to the cascade of the degeneration IVD (). The exposure of MSCs to inflammatory factors (IL-1b and TNF-a) could negatively modify the MSC differentiation potential by promoting osteogen-like mineral deposition, which is not desirable for disk repair (). These findings could provide alternative future therapeutic treatments depending on the administration of specific antagonists of these proteins directly on the disk to prevent pathological proteolisis of the ECM disk. To overcome the temporary effects and obstacles of these factors when applied directly to IVDs, another strategy has been developed that is gene therapy aimed at silencing catabolic pathways or activating anabolic pathways in degenerated IVDs. Genetic therapy has advantages over direct protein delivery. For example, the possibility of sustained long-term effectiveness and endogenous synthesis maintained by growth factors or anti-inflammatory factors (,). The desired nucleic acids are commonly transmitted using a viral vector, so that the functional status of the receptor cells can be modified. For example, an in vitro study has also been established using transfected IVD human cells with adenoviral vectors that transport inhibitor to interleukin-1 (IL-1), an important cytokine in the inflammatory cascade, in degenerated IVD (). The inhibitor effects on IL-1 production were maintained during the 2-week period of the study. Another alternative for the use of gene transfer is to stimulate anabolic pathways. Several studies have shown positive regenerative effects after IVD cell transfection. Transfectious cells have demonstrated a better production capacity of NDE and collagen synthesis II by using genes responsible for BMP-2 production, LIM-1 mineralization protein (LMP-1), Condroitin ABC, TIMP (metalloprotein tissue inhibitor) and SOX9Jeny (-). However, despite their potential security concerns, the potential for immunogenicity and tumorigenesis for this modality needs further research. Another alternative, apart from direct injection of growth factors or their mediated delivery by vectors through gene therapy, is simply the differentiation of primary and direct MSCs during the cult process, in phenotypes similar to IVD with induction by growth differentiation factor 6 (GDF6), bone morphogen protein-2 (BMP-2) and the transformation of growth factor (TGF-β) (). Numerous in vitro and animal studies have portrayed that the TGF-β signaling in internal growth plate and AF cells condrocytes to be critical in the growth and maintenance of the matrix tissue and the cellularity of final plaque cartilage cells (-). Clarke et al. showed that both BM-MSCs and AD-MSCs prepared with GDF6 have demonstrated by identifying NP markers; greater phenotypical differentiation in NP cells with secretion of an NDE that is more proteoglycan rich and consistent with IVD micromechanical properties (). The BMP protein family plays a key role as the growing protein synthesis, the regulation of collagen type II mRNA (). The in vitro application of BMP-2 in a rat model increased cell proliferation and the production of ECM disks (). The IVD rabbits exposed to BMP-7 injections also led to the restoration of disk height and the increase of proteoglycan content (). It summarizes the above studies and shows the effect of different microambients on the growth and survival of stem cells. Table 2 Type of study Cellular sourceIntervention OutcomeHudson et al. ()In vitrohMSCsHypoxic (5% O2) versus normoxic (21% O2)conditionsThe hypoxic expansion of human MSCs improves the 3D ripening of tissues designed IVDsAdesida et al. ()In vitrohBM-MSCsPosic conditions (3% O2) versus normoxic conditions (21% O2)Hypoxic conditions increase the chondrogen potential of hBM-MSCsClarke et al. ()In vitrohAD-MSCs and hBM-MSCsMedia supplemented with TGF-β3, GDF5, or GDF6GDF6 stimulation of AD-MSCs induces differentiation to a phenotype similar to NP and results in a richer proteoglycan matrix with less rigid compositionKumar et al. ()In vitrohMSCsHypoxic (2% O2) cologenic media versus non-thermogenic media MSCs improved the differentiation in NP phenotypes with high levels of expression of aggrecan and collagen II under hypoxic conditions Elabd et al. ()Hypoxic (5% O2) conditions of in vivo human study The radiological assessment of the MRI and the quality of life questionnaire as a result measure showed a significant improvement Stolzing et al. ()In vitroRat nonadherent BM-MSCSDifferent glucose levels in cultures The culture in the middle of high glucose content had a negative effect on the formation of colonies and the differentiation Liang et al. ()In vitrohAD-MSCsAge (mature and young male donor cells), glucose, acidity and osmolarity Low glucose is a positive factor, but high osmolarity and low pH are harmful factors affecting the survival and biological behavior of HAD-MSCs. Age does not affect the results Wuertz et al. ()In vitroRat BM-MSCsAge (mature and young male donor cells), glucose, acidity, osmolarity and combined conditionsThe glucose conditions similar to IVD stimulate the expression aggrecan and collagen-1. Osmolarity and pH strongly decreased the proliferation and expression of matrix proteins. Osmolarity and pH dominated the effects of glucoseMavrogonatou and Kletsas ()In vitroBovine-NP cellsHigh osmolality Liang et al. ()In vitrohAD-MSCsGlucose weight, acidity, high osmolarity and combined conditions Low glucose is a positive factor but high osmolarity and low pH are harmful factors affecting the survival and biological behaviors of AD-MSCsTao et al. ()In vitroRat NPCs, NP-MSCs and co-cultureHigh osmolality High osmolarity inhibits cell viability and decreases the expression of aggrecan and collagen II at levels of MRNA and Wuertz et al protein. ()In vitroRat BM-MSCsAcidityAcidity caused anhibiion of aggrecan, and collagen I, as well as a decrease inproliferation and viability and was associated with a change in cell morphology Purmessur et al. ()In vitroPorcine NCsSynamic RemarkThe ripening of tissues was induced by dynamic hydrostatic pressureYurube et al. () IVD cells in vitroRatStatistic ResearchStotal cell death induced by compression and degeneration See et al. ()In vitroRabbit BM-MSCsExpressive Mechanical StimulationThe extensive remodeling and production of NDEs occurred within the simulated IVDDai et al assembly. ()In vitroRats AD-MSCs Seismic and co-cultive constructions of NPCThe combination of dynamic compression and coculture showed an additive effect on cell differentiation similar to NPLi et al. ()In vitroRat NPCsPriming with rGDF-5rGDF-5 treatment of disc cells from the GDF-5-deficient peroce resulted in adoregulation of the aggrecan and type II collagen genes Chujo et al. ()In vitro and animal in vivo study (rabbit model)Bovine NP and AF cells, rat IVD modelrhGDF-5In vitro, rhGDF-5 increased the DNA and proteoglycan contents. In vivo, rhGDF-5 injection improved disk height, MRI scores and histological classification scores Stoyanov et al. ()In vitrohBM-MSCsTGFß or GDF5 or coculture with bovine NPCs. All groups were incubated at low (2%) or normal (20%) oxygenThe hypoxia and GDF5 led MSCs to the IVD Wehling et al phenotype. ()In vitrohBM-MSCsIL-1beta and TNF alphaBoth IL-1beta and TNF alpha inhibited condrogenesis in a dose-dependent waySteck al. ()In vitrohBM-MSCsTGF beta-3, dexamethasone, and ascorbate After the mediated differentiation of TGF, MSC adopted a gene expression profile that looked like native IVD tissue closer to native Thompson and al articular cartilage. ()In vitroCanine IVD discILGF-1, EGF, FGF, or TGF beta-3TGF beta-3 and EGF obtained greater proliferative responses than FGF; ILGF-1 produced a marginally significant response in the core and no response in the Walsh and other amplifying and transition zone. ()In vivoMurine IVD discGDF-5, TGF beta-3, ILGF-1 or FGFA basic statistically significant increase in disc height 4 weeks after the GDF-5 Li et al treatment was measured. ()In vitroRat AF cellsBMP-2BMP-2 increases the expression aggrecan and collagen type II mRNA. BMP-2 also regulates mRNA expression for cell delivery BMP-7 and TGF beta-3Stem With regard to the delivery of cell-based therapies, the traditional horse of work has been the percutaneous injection assisted by images through the AF. However, transanular injection has raised concerns about damage caused by AF, leaks from the delivery site that induce the formation of osteophytes and the decrease in cell viability due to the shrinking forces caused by small-diameter needles (-). To maximize both the safety and the effectiveness of such a modality, the selection of the needle size and the dose to be delivered should be optimized. Small needle diameters can make it difficult to inject viscous scaffolds such as hydrogels, while larger diameters can induce AF (,). In vivo animal study using a goat model by Gullbrand et al. () a 22G needle was found as safe and feasible without discernible degenerative changes in the MR or histology after 12 weeks. Since the degenerated IVD provides a very limited supply of nutrients, the actual minimum dose of MSC should be determined to maximize post-implantation survival. The largest model study data (canine, pork and ovin) showed that a dose of 106 BM-MSCs/per disk had the lowest number of MSC apoptosis (). Current procedures often show the flow of cells through the injection site and a low rate of survival of the implanted cells. In an attempt to avoid possible AF damage and minimize the extrusion of the injected material, an alternative delivery route that has been suggested is through the péndics (transverse approximation) (). Another alternative is the use of a delivery vehicle to act as injected suspension retainers. A human study in which two patients with IVD degeneration had collagen porous sponges sown with MAM-MSCs autologous implanted by open focus and a collagen sponge was used as a sealant for the surgical hole in IVDs operated. In this study, both patients demonstrated promising results of better results scores and increased hydration of disks as demonstrated by the MR evaluation to 2 years after the intervention (). Other biomaterials such as delivery vehicles, and in particular hydrogels, have been used to overcome retention issues and provide additional support for cell survival and phenotype retention (-) (). An illustration that shows the most common sources used for stem cell harvesting. After the harvest of stem cells are cultivated and framed with growth factors or cultivated in a scaffold for additional support of cell survival. Scale trousers In recent years, various scaffolding materials such as fibrino, hyaluronano or aththecollagen have been developed to maximize the efficiency of delivery of degenerated stem cells (-). Ideally, biomaterial scaffoldings must withstand physiological loads, possess adequate immune compatibility and ability to keep stem cells retained in their construction. A biomaterial scaffolding can promote the feasibility and improve the differentiation of mesenchymal cells in the desired location, providing a three-dimensional 3D microenvironment. A unique difficulty in the implantation and delivery of stem cells is the leak and inability to retain the cells implanted at the site of interest. It has been shown that the injection of a cell suspension in the lumbar rabbit discs has resulted in a 90% loss of the injected cells within the first 30 minutes post injection (). Another concern is the formation of osteophytes as a result of the ectopian osteoblast differentiation through leakages. Thus, the application of a scaffolding material has been strongly recommended to mitigate the risk of leakage and act as a retainer of transplanted cells (). In addition, animal models have established that self-sacrifice BM-MSCs have a relatively short survival time up to 48 weeks after transplant (). Chitosan polysaccharide is a natural component of insect exoskeleton and is both PH and sensitive temperature. An in vitro study showed that chitosan nanoparticles have a powerful anti-inflammatory effect on IVDs degenerated by low-regulation mediators such as IL-6, IL-8, MMP1 and MMP3, while causing the regulation of type II collagen and aggrecan production (). Another in vitro study showed that a chitosan scaffold improved phenotypic differentiation of MSCs to NP as cells with greater production of aggrecans and collagen II (). Atelocollagen, an injectable collagen hydrogel, has been studied in vitro and provides a biocompatible environment that increases NP cell function (-). The in vivo implantation of AF cells sown in athelcollagen scaffolding in rabbit models prevented the progression of IVD space reduction and had viability and proliferative activity (). The gelatin is derived from the collagen treated with thermos and as collagen, induces cell adherence, proliferation and collagen expression II (). The culture of AF cells in a jelly scaffolding showed better cell adherence, ECM protein expression and MMPs inhibition (). An in vivo study showed that MSC-gelatin scaffolding transplant in perforated rabbit inhibited cell apoptosis and maintained the disc height index (). Hyaluronan as scaffolding material was also studied as a potential biomaterial to address difficulties in delivering stem cells. Subhan et al. () in a controlled study on a rabbit model have demonstrated a significant improvement in radiological and histological results in the group of cell-filled scaffolding compared to other control groups. In another controlled study on a damaged IVD ovine model, without cells, implanted resistant to the freezing of the reabsorbable reabsorbable acid implants based on reabsorblic acid were implanted after the nucleotomy of the IVD. Implantation of this implant without polymers induced by NPs tissue regeneration and improvement of the disc water content both radiologically and histologically (). An in vivo sheep model showed greater proteoglycan synthesis of IVD NPs injected with combined MSCs embedded in a fibrin scaffold (). After 6 months, biochemical and histological markers showed better hydration of disk and cellularity in scaffolding and MSCs disks than injected disks with scaffolding alone. In addition, the radiological evaluation revealed better Pfirmann scores compared to the control group. In a prospective study on 15 patients diagnosed with single-level lumbar spondylosis were treated by a percutaneous transplant of encapsulated alogenic juvenile condrocytes in a fibrin matrix (). Six months of follow-up revealed improvements in the hydration of disks by evaluation and improvement of pain and disability scores without reported adverse effects. Porous silk scaffolding has also been studied and it has been shown that they support AF cell attachment and ECM accumulation. Alginate is another natural polymer of polysaccharides derived from algae. Studies on alginate as scaffolding showed differentiation of MSCs induced to cells similar to NP, as well as increased expressions of protein NDE (,). Synthetic polymers have proved promising as a scaffolding material. Examples are polyethylene glucocol (), polycaprolactone (), polyurethane () and polylactic acid (). Restoration of disk height is necessary to ensure the functionality of the IVD. Thus, a multifunctional therapeutic modality has been derived. A recent advance in the field of bioengineering was the combination of such materials to create biphasic scaffolding to design the entire IVD by recapituating the unique structures and functions of both NP and AF (,). For example, a biphasic scaffolding of cells was made in which silk was used for the AF while fibrin and hijaluronan for the NP was generated an entire IVD in vitro (). An in vivo animal study, used a new biophasic IVD-loaded cell by integrating a frozen and linked porcine bone matrix gelatin for the AF and porcine acellular cartilage for the NP (). Another innovative approach to the development of the scaffolding is based on the inherent ability of cells to form their own matrix, much like that of the target tissue (). This approach involves the secultation of cells to produce NDE that will ultimately serve as IVD implant with similar structural characteristics and compositions of native tissue (,). Some researchers also paid attention to natural biological materials such as the IVD decellularized matrix to act as a natural scaffolding for implanted cells. An in vitro study showed potential for the use of the decellularized bovine IVD as an xenogen scaffold (). The studies included in relation to used scaffolding and delivery vehicles for stem cells in the discogenic pain of low back are summarized in .Table 3ReferenceType of studyType of studyType of saleType of saleType of saleType of work Bertram et al. ()In vitro and animal in vivo study (Rabbit model)Rabbit NPCs (cultivated in the fibrin matrix)Injectable cellular fibrin gelUp to 50% of the injected cells of the matrix remained in the core and transition zone in contrast to a rapid loss of medium injected cells. Increased survival of cultivated cells with fibrinaTeixeira et al matrix. ()In vitroBovine IVDChitosan-Diclofenac nanoparticles Chitosan-Diclofenac nanoparticles reduce inflammation and also decrease the degradation of the Richardson NDE and others. ()In vitrohMSCsChitosan-gliceofosfatoChitosan directs MSCs phenotypic differentiation to more NP as Sato et al cells. ()In vitroRabbit AF cells (cultivated in athellocollagen) Panfleet-shaped pamphlet with a membrane seal The amount of collagen type II and its expression of MRNA, GAG and proteoglycans in squamously cultivated cells remained at a higher level than in the cultivated monocapa cells Sakai et al. ()In vitroHuman NPCs (cultivated in athellocollagen)AtelocollagenResults showed that both DNA synthesis and content are significantly greater when cultivated in Atelocollagen than in alginate Yang et al. ()Animal in vivo study (Rabbit model)Rat IVD MSCsPure fibrinous gelatin (PFG)The transplanted MSCs in PFG inhibited apoptosis and slowed down the rate of decreasing the rate of discSubhan et al. ()Animal in vivo study (Rabbit model)Rabbit BM-MSCs (weren't scaffold cultured but were delivered with hyaluronan hydrogel)Hyaluronan hydrogel Best MRI scores for MSCs delivered with hydrogel. The immunohistochemical stain for type II collagen and aggrecan stain were also higherWoiciechowsky et al. ()In vivo animal study (ovin model)Polyliccellular acid (PGA)Histologic analysis showed the growth of cells with typical condrocytic morphology, including cellular distribution, and proteoglycan Chang et al. ()AF VitroBovine Cells (Cultrated in porous silk scaffolding) porous silk scaffolding and grown in dynamic or static flow conditions The dynamic flow conditions and the size of the scaffold pore can affect the formation of AF fabrics designed Yang et al. ()Cells in vivoRat NP and AFAlginate hydrogel seedededed with NPCs, Polycaprolactone (PCL) seededed with AF cellsLong term implantation in rats generated highlyhydrtaated soft tissues and well-integrated into the adjacent vertebraeZeng et al. ()In vivo e in vitrohAD-MSCsPolyacrylate microcryogels (PM)Retention of improved PMs cells delivers assisted cells to a IVDBenz and al mice loading environment. ()In vivo y in vitroHumano IVDHydrogel composed of chemically interlinked albumin by polyethylene glucocol The expression of the mRNAs of cartilage and specific disk remained in hydrogels in vitro and in vivoHu et al. ()In vitroRabbit BM-MSCsSeda/polyurethane (SF/PU) compound hydrogel The compound hydrogel exhibited appropriate physi-mechanical properties such as prosthesis biomaterial for the replacement of NP Kim et al. ()In vivo-coglicliclic acid rat NPCspolylactic As the pores became smaller, the value of the compressive force of the scaffold increased Choy et al. ()Mechanical vitro studyAcellular biphasic pathways made of collagen and glycosaminoglycans (GAGs)Biphasic panels composed of 10 similar fibrous annulus had the best general mechanical performance among the various Elsaadany and al designs. ()Mécnica in vitro studyhAD-MSCsBifasic mechanically conditioned scaffolding encapsulated with hAD-MSCsEchoxial load greater secretion of proteins NDE and expression of AF marker genes compared to non-trained samples Park et al. ()AF vitroPorcine cells (seed wheels)Sea pallet (silver versus porous)Histology, biochemical tests, mechanical tests and gene expression indicated that the scaffolding of the foundry generated more favorable results in terms of expression of NDE and tissue function than the porous scaffolding for AF Xu et al. ()Animal In vivo study (Rat model)Rat NP and AF cells (cultive in the biphasic scaffold in vitro) Biphasic cross-connecting scaffold of the gelatin bone pig matrix for the external AF and the ECM pork acellular cartilage for the construction of NPIVD as tissue formed in mice as confirmed by histology after subcutaneous implants Illien ()In vitroHuman NPC and MSCs (culture with scaffolding)Decellularized injectable bovine ECM material The scaffold maintained the native NP tissue structure and the composition closest to the natural NDE and promoted cellular adaptation of NP and MSCs Current data Stem cell therapy for low-back discogenic pain is still in childhood and will need to demonstrate preclinical efficacy and safety using in vitro and in vivo model systems before being translated into a wide clinical use. One of the greatest challenges facing in vivo animal models is the need to replicate the size and height of the human disk in preclinical trials. For example, the height of the lumbar disc in sheep is approximately 4 mm compared to about 11 mm in humans (). The replication and test of the regenerative potential of stem cells under conditions that mimic the microenvironment complex in the IVD is another challenge. Not only biochemical conditions, but also physiological load conditions must be reproduced to allow reproducible and reliable results after the transplant. The physical environment can be imitated through sectation in soft 3D scaffolding as hydrogels and mechanical tensions such as dynamic compressive loads or hydrostatic pressures applied through bioreactors (-). The high cost of clinical trials and ethical concerns regarding the use of embryonic and umbilical stem cells is another obstacle. A recent systematic review showed that most literature available on the subject is methodologically poor with small sample sizes, high risk of bias and lack of control intervention (). Equally important, the measure of result reported by the current patient is influenced by significant placebo effects (). Also ensure safety and tolerance mandates that uncontrolled differentiation of stem cells is reviewed and developed more efficient execution strategies. Another challenge is the correct goal of painful degenerate disks instead of painless degeneration. Except in cases with nerve root compression or central stenosis, conventional MRI studies do not effectively distinguish between painful and non- painful degenerators (). In addition, to support the clinical application and effectiveness of stem cell therapy, there is a critical need for new techniques to quantify therapeutic effects at treated levels. The most recent techniques of magnetic resonance, such as relaxation times T1ρ and T2, and the transfer of chemical exchange saturation (CEST) provides a quantitative analysis for the composition of disk indicating more accurately DDD (,). Painful disks are characterized by hypoxia and inflammation that leads to the accumulation of certain metabolites such as lactates, alaninos and lipids (). These metabolites can serve as biomarkers that are detectable through MR spectroscopy (). A recent imaging technique is the quantitative MRI of sodium that detects sodium levels in the IVD and therefore the state of hydration. A recent study suggests that this technique can differentiate discs between asymptomatic and symptomatic patients (). However, sodium magnetic resonance is used in a limited way due to the need for special hardware modifications to the conventional magnetic resonance imaging scanner. It has been shown that the emission tomography of fluorodeoxiglucosa 18 fluorodeoxiglucosa fluorodeoxiglucosa (FDG-PET) identifies inflated degenerate disks and can be a beneficial diagnostic tool (). As indicated above, inflammation is a central feature of painful disks. PET/CT can be useful in patient selection and location of spinal levels. Invasive provocative discography, which includes discal stimulation and morphological evaluation, is often used to distinguish a painful disk from other potential sources of pain such as facet joint pain. A recent systematic review supported the use of provocative lumbar discography as a precise diagnostic tool (). ConclusionsRegenerative therapy of stem cells novels for discogenic back pain is a promising alternative to conventional surgical management and other non-operational alternatives. The selection of patients, as well as the precise location of pain generators, is a prerequisite for successful effective treatment. The stem cells used are derived widely from the IVD itself (mother cells or resident primary cells) or are derived from other pluripotent sources such as MB or adipose tissue. Many challenges face this relatively childish field, ranging from isolation, culture and the handover of hosts. The degenerated IVD complex and hard microenvironment plays a harmful role in the multiplication and survival of transplanted stem cells. More basic scientific and clinical studies are needed to establish the clinical effectiveness of such treatments. Limitations This is a bibliographic review of current concepts and advances in the field of regenerative stem cell therapy for discogenic back pain. Most of the available studies are animal or in vitro studies with relative shortage of human tests. However, a force of our review is that we perform a complete search of multiple databases. Independent evaluators conducted the search, selected and evaluated the studies included. AcknowledgementsNone.NotesEtica Declaration: The authors are responsible for all aspects of the work to ensure that issues related to the accuracy or integrity of any part of the work are properly investigated and resolved. FootnotesConflicts of interest: The authors do not have conflicts of interest to declare. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
Stem cell injections for axial back pain: a systematic review of associated risks and complications with a diffuse hyperplastic gliosis illustration that results in echine rubber syndrome Buy now Spine - 1 year subscription (only individual) JNS + Pediatrics + Spine - 1 year subscription (only individual) Buy now Spine - 1 year subscription (only individual) JNS + Pediatrics + Spine - 1 year subscription (only individual) OBJECTIVE The low back axial pain is a disease of epidemic proportions that carries a heavy global burden on the active labor force and results in more than half a billion dollars in annual costs. The injections of stem cells are increasingly announced as a restorative solution for various degenerative diseases and are becoming more affordable and accessible by the public. In the media, multiple reports have been submitted that these injections are readily available abroad outside of clinical trials, but the scientific evidence supporting them remains low. The authors present a case of serious complication after a stem cell injection for back pain and provide a systematic review of the literature of the efficacy of this treatment, as well as associated risks and complications. The systematic review of METHODSA literature was carried out using the PubMed, Google Scholar and Scopus electronic databases to identify articles that report stem cell injections for axial back pain in accordance with PRISMA guidelines. Primary care focused on results and complications. A case of glial hyperplasia of the roots of the equina caulk is also reported directly related to the injections of stem cells carried out abroad. RESULTS The authors identified 14 publications (including a total of 147 patients) that met the search criteria. Three of the articles presented data for the same patient population with different follow-up durations and were analyzed as a single study, reducing the total number of studies to 12. In these 12 studies, follow-up periods ranged from 6 months to 6 years, with 50% follow-up of 1 year or less. Most of the studies reported favorable results, although 36% used subjective measures. There was a tendency of pain relief to disappear after 6 months to 2 years, with patients seeking a surgical solution. Only 1 study was a randomized controlled trial (RCT). CONCLUSIONS There is still insufficient data to support stem cell injections for back pain. Additional RCTs with long-term follow-up are required before statements on effectiveness and security can be made. Spine - 1 year subscription (only individual) JNS + Pediatrics + Spine - 1 year subscription (only individual) Spine - 1 year subscription (only individual) JNS + Pediatrics + Spine - 1 year subscription (only individual) Contributor NotesINCLUDE WHEN CITING Published online September 6, 2019; DOI: 10.3171/2019.6.SPINE19594.Disclosures The authors do not report any conflict of interest regarding the materials or methods used in this study or the conclusions specified in this document. Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L: brain tumor derived from the donor after the transplant of neuronal stem cells in a telangiectasia ataxia patient. PLoS Med 6:e1000029, 2009American Diabetes Association: Economic costs of diabetes in the United States in 2017. Diabetes care 41:917–928, 2018 Andersson GB: Epidemiological characteristics of low back chronic pain. Lancet 354:581–585, 1999 Becker AJ, McCulloch EA, Till JE: Cytological demonstration of the clonal nature of the stem colonies derived from transplanted mouse marrow cells. Nature 197:452–454, 1963 Centeno C, Markle J, Dodson E, Stemper I, Williams CJ, Hyzy M, : Treatment of degenerative lumbar pain of disco disease associated with autoculcated mesenchymal stem cells: a pilot study on safety and efficacy. J Transl Med 15:197, 2017Comella K, Silbert R, Parlo M: Effects of intradiscal implantation of the most plasma-rich estromal vascular fraction in patients with degenerative disco disease. J Transl Med 15:12, 2017de Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A, : The association between the degeneration of the lumbar disc and the pain of the back: the influence of the age, gender and individual radiographic characteristics. Spine (Phila Pa 1976) 35:531–536, 2010Elabd C, Centeno CJ, Schultz JR, Lutz G, Ichim T, Silva FJ: Intra-discal injection of mesenchymal stem cells cultivated by autochthonous bone marrow and hypoxic in five patients with chronic lower back pain: a long-term safety and feasibility study. J Transl Med 14:253, 2016 Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V: Mesenchymal stem cell therapy of osteoarthritis: current knowledge and future prospects. Biomed Pharmacother 109:2318–2326, 2019 Haufe SM, Mork AR: Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate intervertebral disks. Stem Cells Dev 15:136–137, 2006Hurst RW, Bosch EP, Morris JM, Dyck PJ, Reeves RK: Inflammatory hypertrophic cauda equina following intrathecal neural th cell injection. Muscle Nerve 48:831–835, 2013 (Erratum in Muscle Nerve 49:298–303, 2014)Kirkland EB, Heincelman M, Bishu KG, Schumann SO, Schreiner A, Axon RN, : Trends in health expenses among American adults with hypertension: national estimates, 2003–2014. J Am Heart Assoc 7:e008731, 2018Kumar H, Ha DH, Lee EJ, Park JH, Shim JH, Ahn TK, : Safety and tolerability of the combined intradiscalo implant of adipose mesenchymal stem cells and hyaluronic acid in patients with chronic pain of low discogenic back: 1 year follow-up of a study phase. Stem Cell Res Ther 8:262, 2017Lee JW, Shin HI, Park SY, Lee GY, Kang HS: Therapeutic Test for Injection of Interlaminant Fluoride Steroids for Low-Axial Back Pain: Predictors of Effectiveness and Results. AJNR Am J Neuroradiol 31:1817-1823, 2010Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA: Treatment of stem cells from degenerative eye disease. Stem Cell Res (Amst) 14:243–257, 2015 Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ: Clinical experience in cell-based therapeutics: Disc condrocyte transplant A treatment for degenerated or damaged intervertebral disk. Biomol Eng 24:5–21, 2007 J Backpack, Sakai D, Nakamura Y, Watanabe T, Yamamoto Y, Kato S: Intervertebral-disc repair with activated nucleus cell transplant: a three-year prospective clinical trial of safety. Eur Cell Mater 29:202–212, 2015 Noriega DC, Ardura F, Hernández-Ramajo R, Martín-Ferrero MA, Sánchez-Lite I, Toribio B, : Repair of intervertebral disk by alogénic bone marrow mesenchymal cells: a randomized controlled trial. Transplantation 101:1945–1951, 2017 Oehme D, Goldschlager T, Ghosh P, Rosenfeld JV, Jenkin G: Cell-based Therapies Used to Treat Disc Degenerative Lumbar Disease: A Systematic Review of Animal Studies and Human Clinical Trials. Stem Cells Int 2015:946031, 2015Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J: Repair of intervertebral disks by autological mesenchymal bone marrow cells: a pilot study. Transplant 92:822–828, 2011Pang X, Yang H, Peng B: Human cord mesenchymal stem cell transplant for the treatment of low back chronic pain. Medical pain 17:E525–E530, 2014 Pettine K, Suzuki R, Sand T, Murphy M: Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimal follow-up of two years. Orthop 40:135–140, 2016 Pettine KA, Murphy MB, Suzuki RK, Sand TT: Percutaneous injection of autologous bone marrow concentrated cells significantly reduces lumbar discogenic pain over 12 months. Mother cells 33:146–156, 2015 Pettine KA, Suzuki RK, Sand TT, Murphy MB: Intradiscal injection of autologic bone marrow concentrate for the treatment of degenerative disk disease with triennial tracking. Orthop int 41:2097–2103, 2017Rice CM, Scolding NJ: adult stem cells: reprogram neurological repair? Lancet 364:193–199, 2004 Rivera CE: Lumbar epidural steroid injections. Phys Med Rehabil Clin N Am 29:73–92, 2018 Vadalà G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD: Mesenchymal stem cells inject into degenerated intervertebral disk: cell leakage can induce the formation of osteophytes. J Tissue Eng Regen Med 6:348–355, 2012Yim RL, Lee JT, Bow CH, Meij B, Leung V, Cheung KM, : A systematic review of the safety and effectiveness of Mesenchymal stem cells for the degeneration of the disk: ideas and future directions for regenerative therapeutics. Stem Cells Dev 23:2553–2567, 2014Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y: Regeneration therapy of discs with mesenchymal stem cell transplant: a report of two case studies. Spine (Phila Pa 1976) 35:E475–E480, 2010 Metrices All the time Last year After 30 days Abstract views 1675 1452 645 Full text views 103 68 6 PDF Downloads 139 56 5 EPUB Downloads 0 0 0 © Copyright 1944-2021 American Association of Neurological Surgeons Powered by PubFactory Limitation of characters 500/500 Limitation of characters 500/500

Chronic back pain stem cell treatment could cut need for opioids | New Scientist
Regenexx Stem Cells for Back Pain in Bellevue and Seattle
Multipotent Mesenchymal Stem Cell Treatment for Discogenic Low Back Pain and Disc Degeneration
Does Stem Cell Therapy Work For Back Pain? - Regenexx
Stem Cells For Back Pain | Stem Cells For Herniated Discs
Cell Therapy for Back Pain | A Natural Treatment
Stem Cells For Back Pain | Stem Cells For Herniated Discs
Does Stem Cell Therapy Work For Back Pain? - Regenexx
If you've had a stem cell treatment, how was your experience? | The Niche
Stem Cells For Back Pain | Stem Cells For Herniated Discs
What's New In Treating Chronic Back Pain? Doctors Investigate Stem Cell Therapy - YouAreUNLTD
Lower Back Pain | Causes, Treatments, Exercises, Back Pain Relief
Stem cell injections for axial back pain: a systematic review of associated risks and complications with a case illustration of diffuse hyperplastic gliosis resulting in cauda equina syndrome in: Journal of Neurosurgery:
Stem Cells in the Treatment of Disease | NEJM
Stem Cell Treatments Flourish With Little Evidence That They Work - The New York Times
Cendant Stem Cell Center Review - Read BEFORE You Go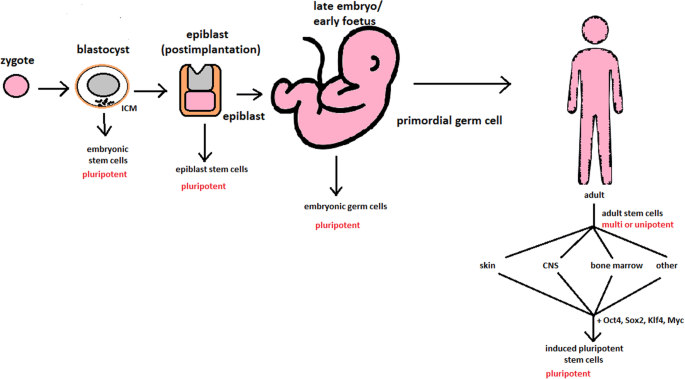
Stem cells: past, present, and future | Stem Cell Research & Therapy | Full Text
Stem cell injections for axial back pain: a systematic review of associated risks and complications with a case illustration of diffuse hyperplastic gliosis resulting in cauda equina syndrome in: Journal of Neurosurgery:
Stem cell clinics' much-hyped treatments lack scientific support | Science News
Back Pain: Stem Cell Treatments May Help
How To Avoid Invasive Back Pain Treatments with Stem Cells
Pin on cord blood banking reviews
Does stem cell therapy for knee meniscus tears and post-meniscectomy work? – Caring Medical Florida
Cell‐based therapy to reduce mortality from COVID‐19: Systematic review and meta‐analysis of human studies on acute respiratory distress syndrome - Qu - 2020 - STEM CELLS Translational Medicine - Wiley Online Library
Stem Cell Herniated Disc - Should You Get Your Disc Injected with Stem Cells? - Regenexx Blog
Stem Cells For Back Pain | Stem Cells For Herniated Discs
Stem Cell Therapy: Review and Future Prospects (Updated Aug 2020)
Low back pain - Wikipedia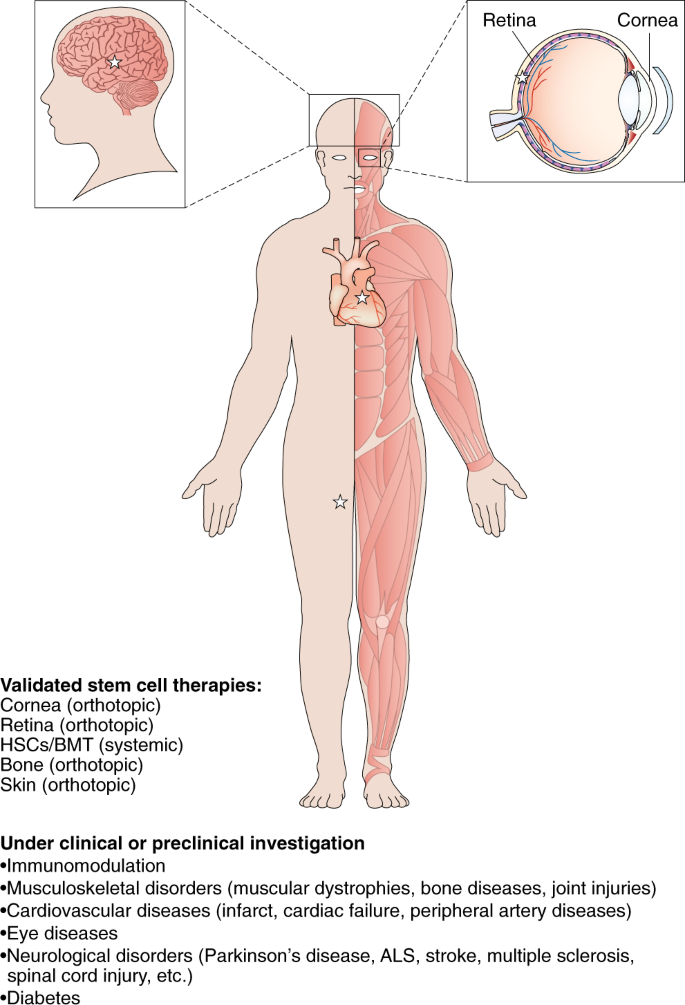
Advances in stem cell research and therapeutic development | Nature Cell Biology/cdn.vox-cdn.com/uploads/chorus_asset/file/13676526/GettyImages_603576872.jpg)
Stem cell therapy: FDA investigates clinics offering unproven treatments - Vox
Osteoarthritis and stem cell therapy in humans: a systematic review - Osteoarthritis and Cartilage
Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis - EClinicalMedicine
Stem Cells in the Treatment of Disease | NEJM
Stem Cells for Knee Injuries Joint Arthritis & Knee Pain
5 Benefits of Stem Cell Therapy: Advanced Spine and Pain: Orthopedic Specialists
Chronic back pain stem cell treatment could cut need for opioids | New Scientist
knee pain treatment Portales NM | Stem Cell doctors Portales NM | by matthew ess | Medium
Okanagan man says stem cell therapy changed his life – Summerland Review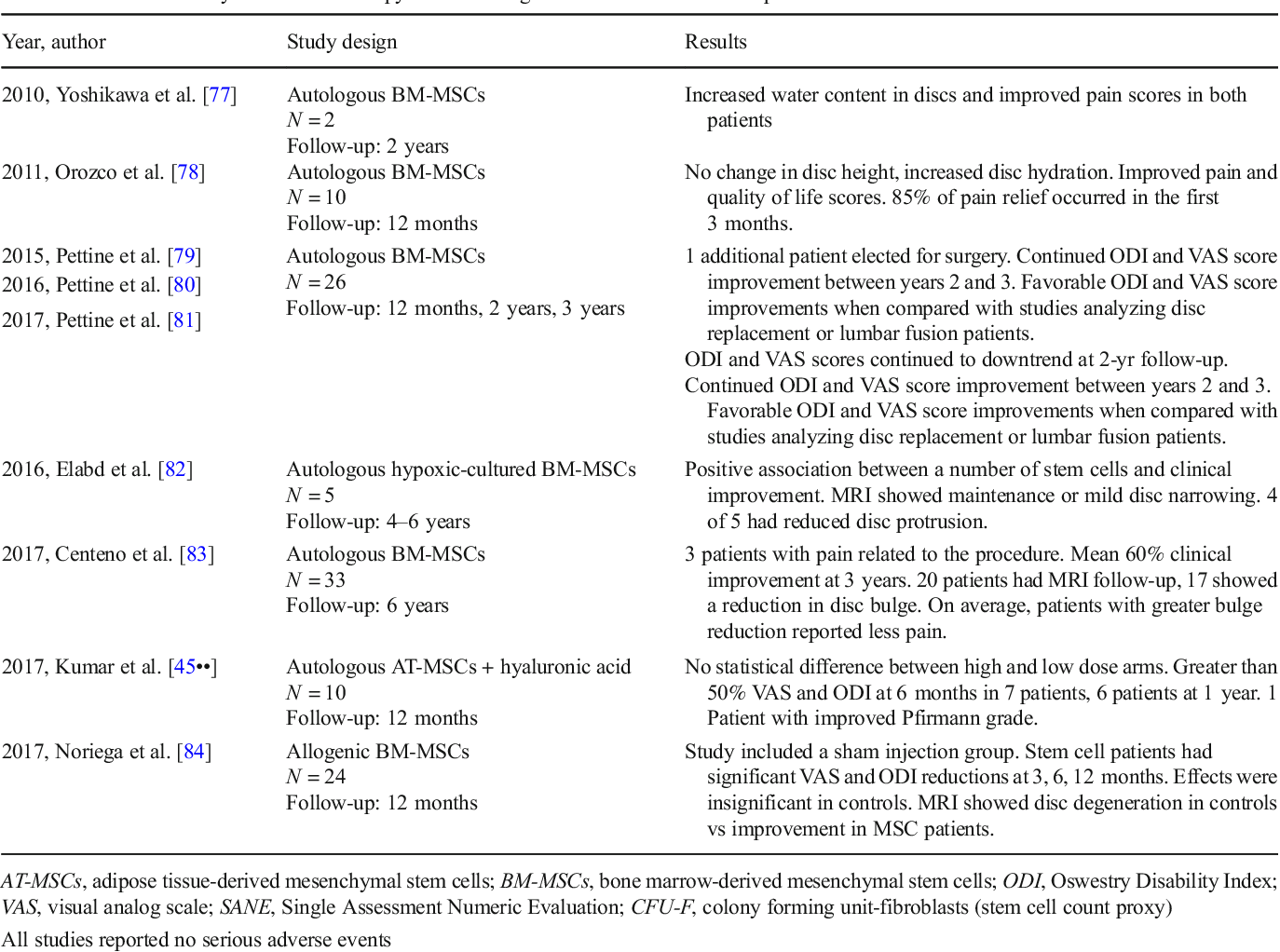
Stem Cell Therapies for Treatment of Discogenic Low Back Pain: a Comprehensive Review | Semantic Scholar
 Does Stem Cell Therapy Work For Back Pain? - Regenexx
Does Stem Cell Therapy Work For Back Pain? - Regenexx




























/cdn.vox-cdn.com/uploads/chorus_asset/file/13676526/GettyImages_603576872.jpg)









Posting Komentar untuk "stem cell therapy for back pain reviews"